DFG Research Unit FOR 2685 | B3
The impact of bacterial activity on decay and fossilization of arthropods: An experimental approach
Abstract
The objective of this project is to improve the general understanding of decay and fossilization processes in context with microbial activity. To this end we will monitor the disarticulation of whole arthropods and characterize the developing of microbial communities on the carcass over time. The experiments will not only observe the physical disarticulation, but aim to understand the influence of biological, chemical and physical parameters and to identify the best conditions for mineralization.
Insights into evolution and phylogeny of arthropods are derived from excellently preserved fossils, which were formed by a complex sequence of biological, chemical and geological processes that starts immediately post mortem. Between death and embedding, decay (aerobic) and putrefaction (anaerobic) catalyzed by microorganisms that originate from the gut flora of the arthropod and its environment, may lead to a partial loss of characteristic features important for phylogenetic interpretations [1-3]. In addition to the decay of organisms, bacteria have also been shown to contribute to the formation of fossils, by synthesizing biofilms [4] which later fossilize by precipitation of minerals and yield casts or pseudomorphs of the hard and fine tissues of the carcass [5-8]. Also the precipitation of minerals might be a product of bacterial activity [9].
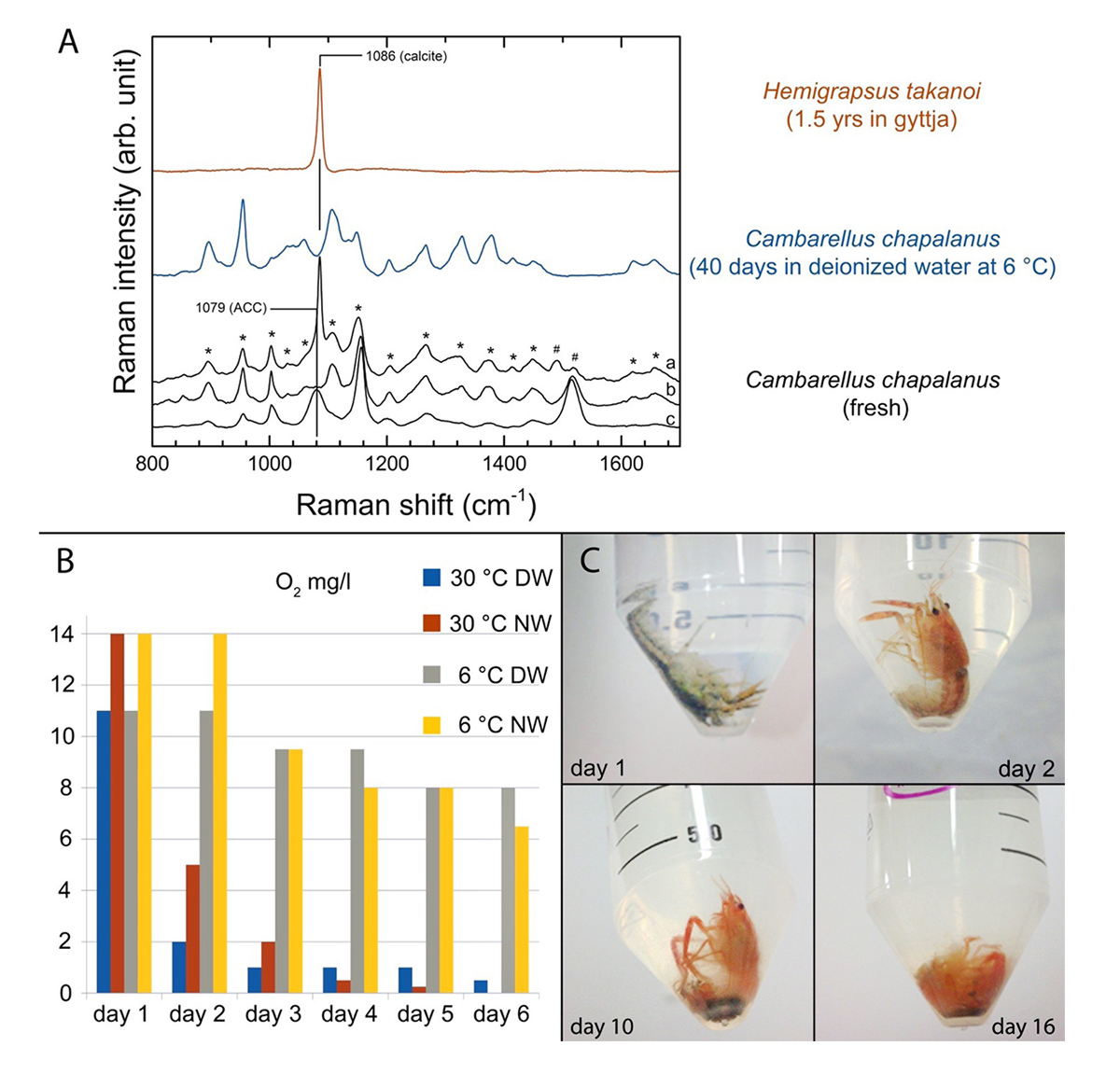
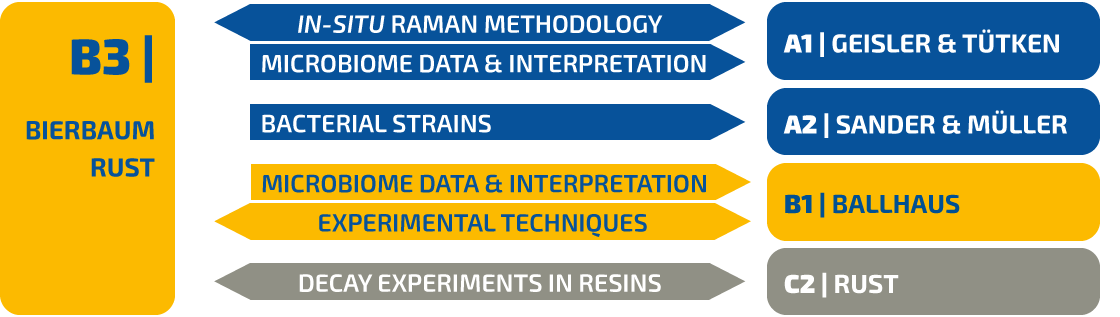
Figure 1 | (A) Chitin degradation (  fused lines of Carapace Cuticle Layers (CCL) of Hemigrapsus takanoi, embedded in mud flat sediment for 1.5 years;
fused lines of Carapace Cuticle Layers (CCL) of Hemigrapsus takanoi, embedded in mud flat sediment for 1.5 years;  molecular com-position of CCL of C. chapalanus on day 11 [a: Epicuticle; b: Exocuticle; c: Endocuticle],
molecular com-position of CCL of C. chapalanus on day 11 [a: Epicuticle; b: Exocuticle; c: Endocuticle],  fused lines of CCL on day 40 in DW at 6 °C).
(B) Oxygen saturation during the decomposition of C. chapalanus for the first 6 days (DW: Deionized Water; NW: Natural Water).
(C) Decomposition of C. chapalanus in NW at 30 ° C.
fused lines of CCL on day 40 in DW at 6 °C).
(B) Oxygen saturation during the decomposition of C. chapalanus for the first 6 days (DW: Deionized Water; NW: Natural Water).
(C) Decomposition of C. chapalanus in NW at 30 ° C.
A preliminary decay experiment showed that disarticulation of the crayfish Cambarellus chapalanus was fast (Fig. 1C). Both studied specimens harbored an individual gut microbiome including symbionts. In contrast, the microbiome of the decaying crayfish derived mostly from the habitat. However, after two weeks, oxygen was depleted and more anaerobic species deriving from gut or soil were detected (Fig. 2 and Fig. 3). After 40 days in water no calcium carbonate could be found in the molecular structure of an isolated cuticle sample of C. chapalanus (Fig. 1A). In contrast, the cuticle of Hemigrapsus takanoi seemed to consist only of calcite after being embedded in mud flat sediment for 1.5 years.
Figure 2 | Microbiome of the gut of crayfish and decaying crayfish: Percentages of bacterial families in the samples. S: Sample; TW: Tank Water.
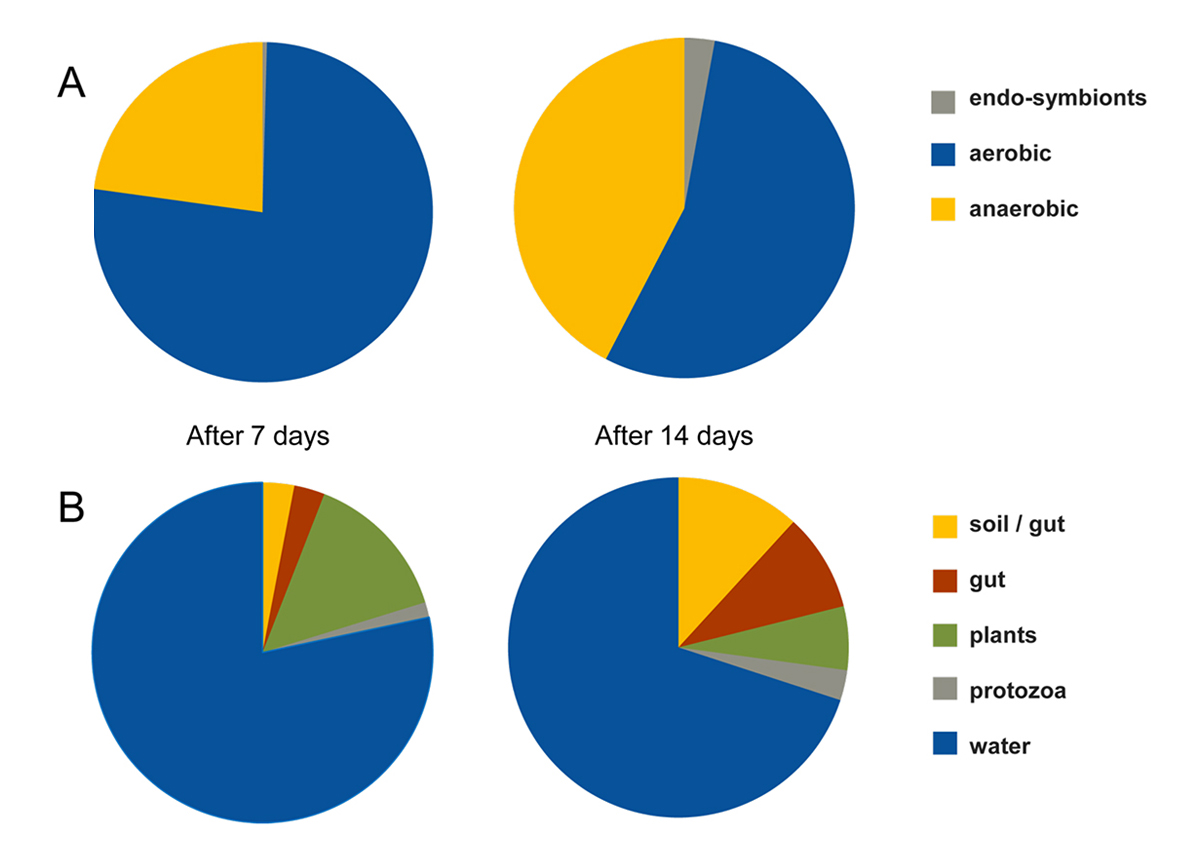
Figure 3 | (A) Percentages of aerobic vs. anaerobic bacteria after one and two weeks of decay. (B) Assignation of the genera present in the microbiome to different habitats.
- Decay of arthropods is mediated by bacteria that are strong producers of exoenzymes and is modulated by environmental conditions. Here, extrinsic bacteria are more relevant in the decomposition process than intrinsic bacteria.
- Formation of biofilms may lead to pseudo-morphs that may undergo fossilization.
- The majority of bacteria shows a different behavior under different environmental conditions.
- The morphology of arthropods, their grade of sclerotization and lifestyle (e.g. feeding, behavior), is leading to different patterns of decay.