DFG Research Unit FOR 2685 | A1
Experimental alteration of bones and teeth: Quantification of effects on inorganic and organic Compounds
Abstract
This project aims to experimentally identify, understand, and essentially quantify the chemical and structural changes associated with various reaction steps underlying the degradation (organic phase decay, bioapatite dissolution) and preservation (bioapatite recrystallization, mineralization) of bones and teeth in aqueous solutions from the macroscopic down to the atomic length scale. This shall be achieved by the use of advanced bulk- to nano-analytical techniques to characterize the run products as well as the solutions of multi-isotope tracer experiments with bone and dental tissues in aqueous solutions under both sterile and microbially inoculated conditions.
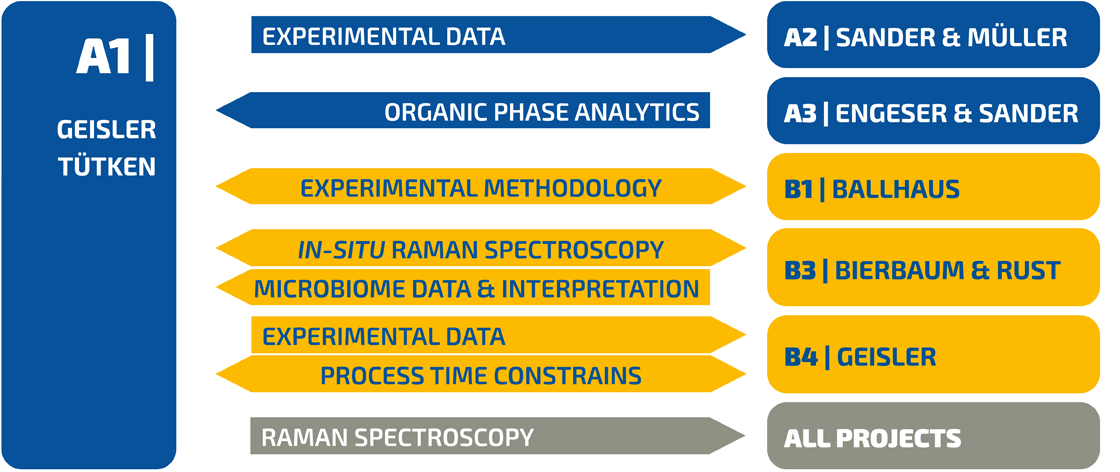
The stable and radioactive isotope systems in fossil bones and teeth represent important geochemical archives to reconstruct the palaeobiology, palaeodiet, and palaeoenvironment of fossil vertebrates (Fig. 1) [1]. Bones and teeth are composed of nano-crystalline, carbonated bio- apatite embedded in a collagen matrix, both of which are prone to alteration during fossilization [1-4]. The process of bone and tooth fossilization can be split into two main, albeit overlapping phases with an initial phase of collagen degradation followed by the chemical and structural modification of the bioapatite. In nature, both steps are usually accompanied by the mineralization of the vascular and osteocyte space [2]. The transport and reaction mechanisms underlying these fossilization processes are not yet fully understood, in particular not at the mesoscopic to atomic scale.
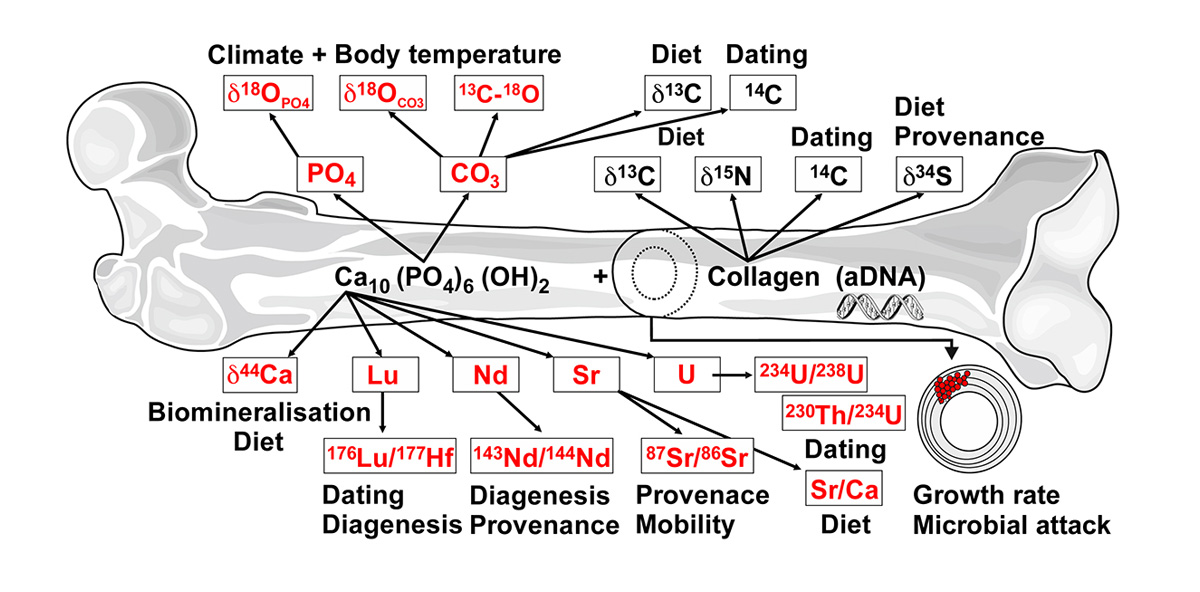
Figure 1 | Elements and isotopes in bones (the same applies for teeth) and their applications for palaeoenvironmental and palaeobiological reconstructions [1]. Those geochemical proxies that are in the focus of this project are marked in red.
As feasibility test, first fossilization experiments were performed with ostrich bone at temperatures between 90 and 190°C for 14 days in an acidic solution, containing 70 ± 2 ppm of all rare earth elements (REEs) and U, as well as 32 other elements [5]. The solution result of these experiments demonstrated a first order temperature dependence of the incorporation of REEs and U into bioapatite. Laser ablation inductively-coupled plasma mass spectrometric measurements across the altered bone samples further revealed a REE and U enrichment in the outer parts with decreasing concentrations towards the interior [5]. In a second feasibility study [6], the mechanism and kinetics of fluorine incorporation in teeth was studied at 21°C by novel in-situ, real-time, and confocal Raman experiments with elephant tooth dentin immersed in solutions with different NaF concentrations (Fig. 2). These short-term experiments demonstrated that it is possible to partly fluoridate the bioapatite in the outer ~50 μm of dentin at room temperature only within a few days. Both studies demonstrated the importance of the initial alteration processes for the long-term preservation of bones and teeth.
Figure 2 | Raman spectroscopic results from an in-situ, real-time experiment with tooth dentin and a 0.5M NaF solution at 21°C. (a) False color Raman images of the v1(PO4) mode frequency of dentine apatite as a function of space and reaction time (in h). (b) Frequency and (c) the full width at half maximum (FWHM) of the v1(PO4) band as a function of time. (d) The FWHM as a function of the frequency.
By combining systematic multi-isotope tracer experiments with novel analytical techniques, having a sub-micrometer spatial resolution, this project aims to
- identify and investigate the reaction mechanisms and transport processes during bone and tooth taphonomy, including the preservation and decomposition of the organic matrix and the impact of micro-organisms.
- quantify the T-t-Xsol dependences of the identified reaction steps.
- assess the resistance of the in vivo stable isotope composition and the organic matter against diagenesis.