DFG Research Unit FOR 2685 | B1
Physico-chemical and microbial processes governing the decay of organic substance in sediment under anoxic conditions
Abstract
The aim of this project is to simulate a fossilization of a fish and starfish experimentally. To achieve this, the carcass will be embedded into sediment and covered with seawater. The atmosphere this seawater is in contact with will be artificially adjusted to promote the speed of fossilization and the formation of pyrite. This experiment will be monitored very closely. The pH and Eh of the overlying seawater and the pore water around the carcass will be measured continuously. Also the chemical composition of the sediment and the fish will be analyzed regularly. A key aspect of this project is to understand the compositional mobilization and migration within this “decay box”.
It has been well established, that it is an important prerequisite for fossilization (especially for exceptional preservation) to isolate a carcass from being consumed by scavengers. This can either happen through sinking or covering by turbidity currents. There have been a number of important experimental taphonomic studies that address the processes that happen after burial and ultimately lead to permineralization of organic tissue [1-7]. These studies established, that the chemical conditions of a system change dramatically during fossilization in terms of ion content in the surrounding solution as well as in terms of pH. But what is still missing are experimental studies that try to duplicate fossilization under strictly anoxic to euxinic conditions, while continuously monitoring the electro-chemical parameters of the whole system (carcass/ fossil, pore water and overlaying seawater) whilst fixing the inorganic parameters as closely as possible. These pioneering studies are an excellent basis onto which to build the experimental approach followed here.
We introduced to the field of low temperature petrology a novel experimental technique that simulates the interaction of a gas atmosphere with seawater under varying pH- and Eh-conditions (Figs.1-3) [8]. This technique allows us to directly monitor the electro-chemical properties of a seawater-atmosphere system whilst conducting precipitation experiments in said system. In our previous work these experiments have been strictly abiotic/inorganic. Their aim was to simulate the formation of siderite in Archaean Banded Iron Formations (∼2.4 Ga). Obviously, experiments in sediment are more demanding, but these previous works will help a lot in handling this more complex, yet very similar, system.
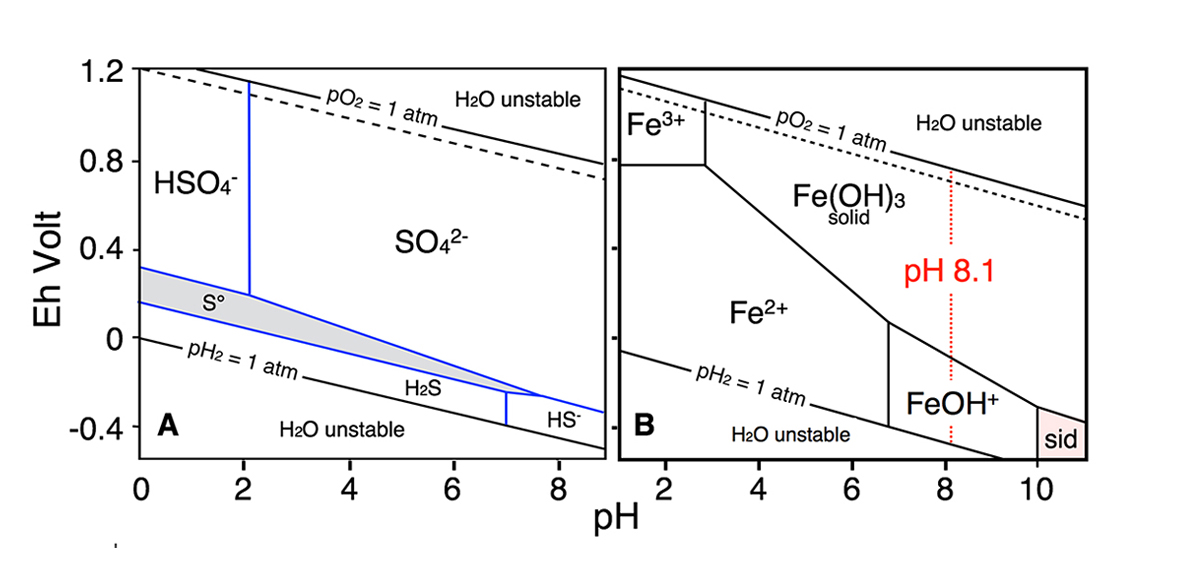
Figure 1 | Phase diagrams of the H-S-O and Fe-C-O-H systems to display the relative stabilities of S and Fe species in aqueous solutions as a function of Eh and pH.
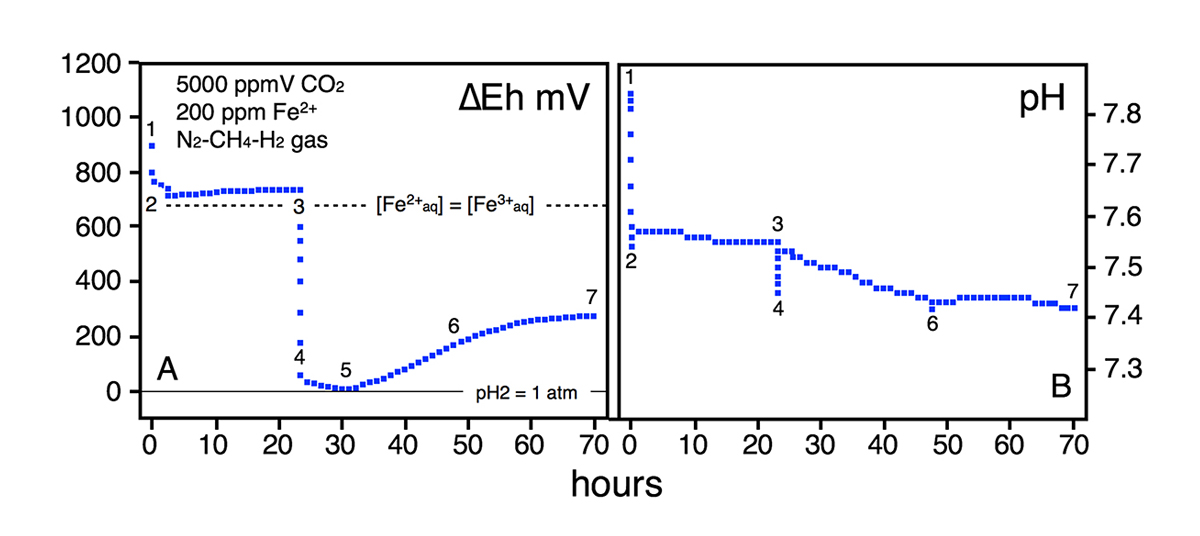
Figure 2 | Reaction progress with respect to ∆Eh and pH within a solution with 200 ppm Fe2+ equilibrated with 5000 ppmV CO2 in a reduced N2-H2-CH4 gas matrix.
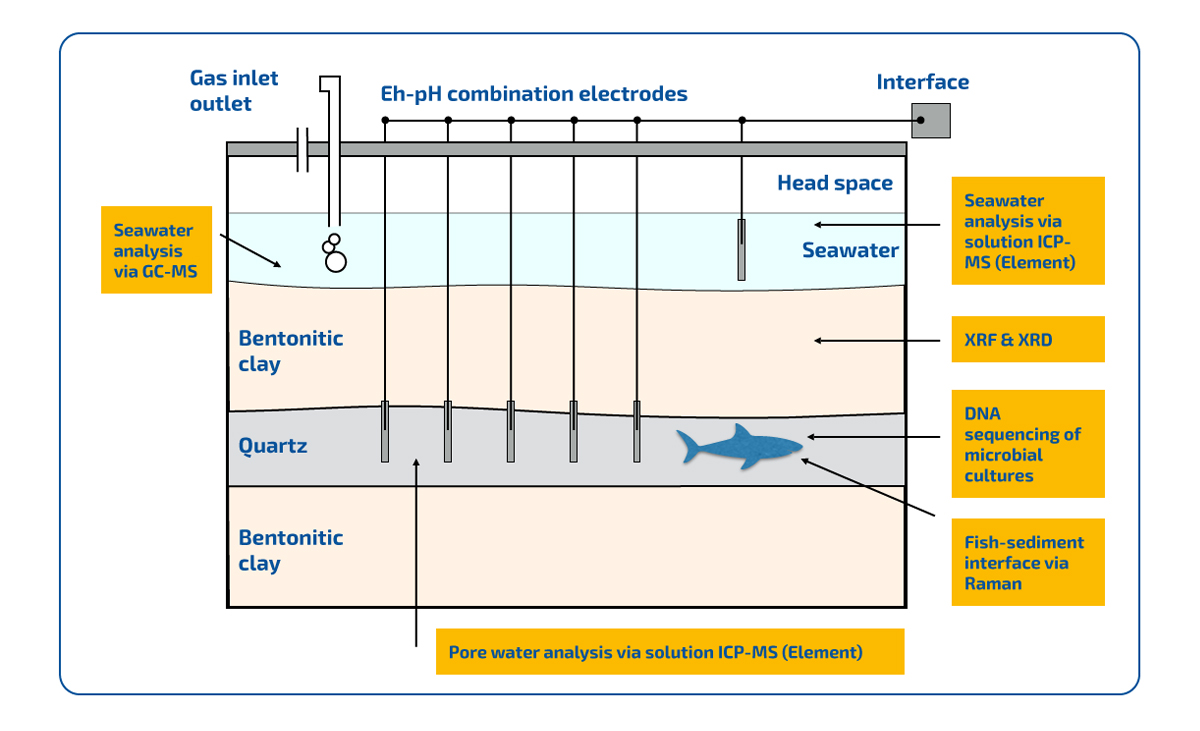
Figure 3 | Schematic experimental setup. The basin is designed as described in the abstract. The Eh and pH of the seawater are continuously monitored via combination electrodes. In the yellow boxes we describe the different analytical methods, that are used on the respective parts of the whole experiment.
The general goal of this project (FIg.4) is to introduce quantitative aspects (such as thermodynamics and experimental approaches), common in experimental petrology, into the field of experimental taphonomy. More specifically we want to explore how the inorganic conditions of a system evolve while fossilization processes take place. We want to check how pH- and Eh-values in the sediment react to the decay of an organism. Also the bacterial communities that evolve around a carcass (as a function of time) are of great interest, since they might be crucial in the process of permineralization of organic tissue.
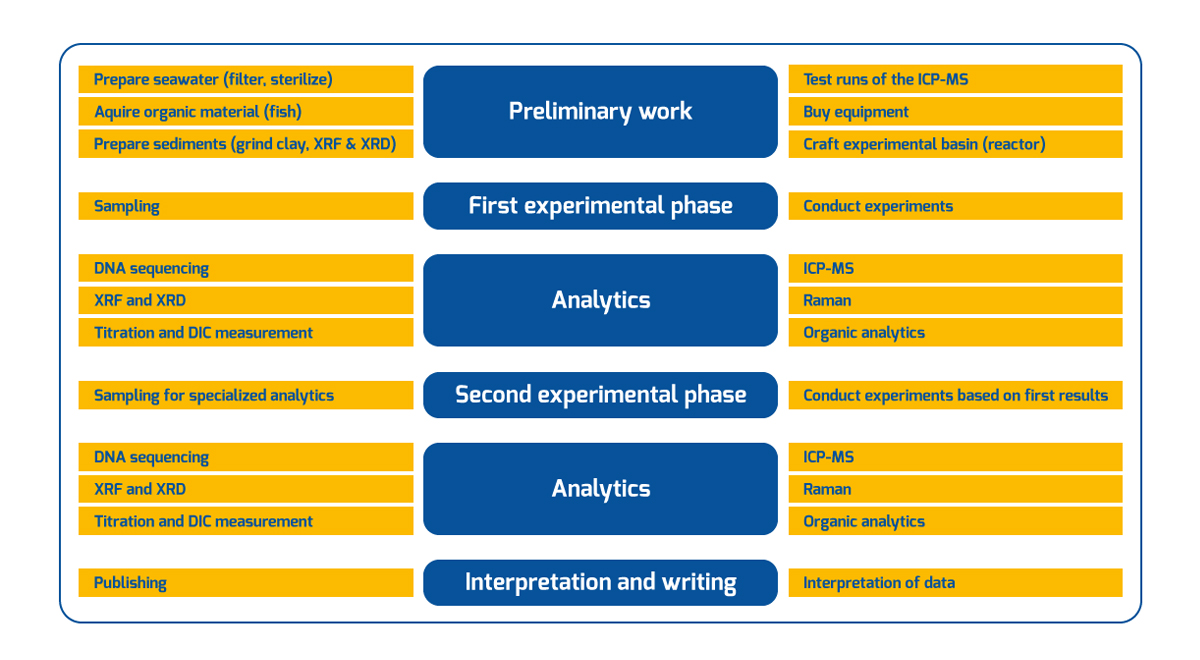
Figure 4 | chematic workflow that shows the tasks and analytical procedures to be conducted at the different phases during the project. (Klick to entlarge)